Letrozole is an oral non-steroidal aromatase inhibitor approved for the treatment of hormonally responsive breast cancer after surgery. It has been used (off-label) for ovulation induction as part of the IVF process since 2001.
 How do aromatase inhibitors work?
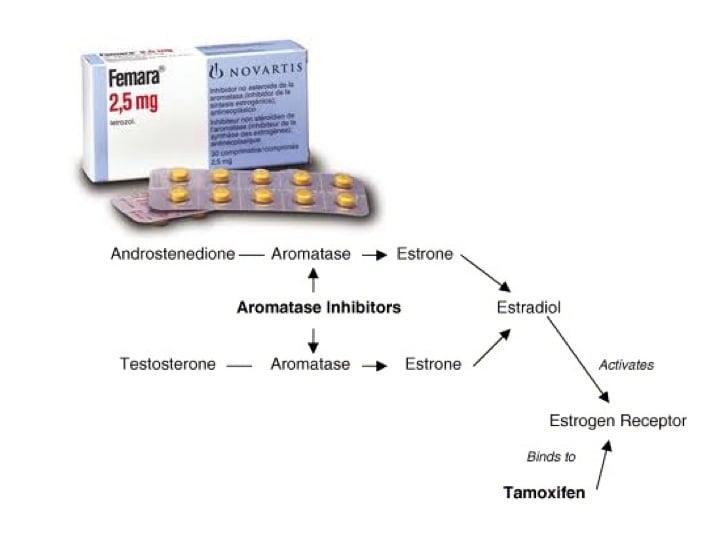
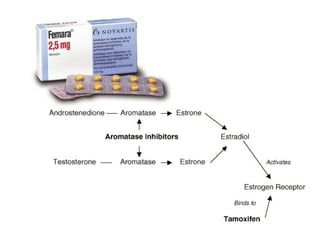
How do aromatase inhibitors work?Estrogens are produced by the conversion of androgens through the activity of the enzyme aromatase. Estradiol (E2, the relevant estrogen) produced by the ovary in turn, exerts a negative feedback effect (inhibits) on follicle stimulating hormone (FSH) release from the hypothalamic-pituitary axis (in the brain).
When Letrozole blocks aromatase activity, there is a drop in E2 levels and release of the hypothalamic/pituitary axis from estrogenic negative feedback. The resultant increase in FSH secretion stimulates growth of ovarian follicles.
Because AIs do not deplete estrogen receptors, as does clomiphene citrate, normal central feedback mechanisms remain intact. As the dominant follicle grows and estrogen levels rise, normal negative feedback occurs centrally, resulting in suppression of FSH and atresia of the smaller growing follicles. A single dominant follicle, and mono-ovulation, should occur in most cases.
With aromatase inhibition, there is a temporary increase in androgen levels in the ovary. Androgens are known to increase the sensitivity of the follicles to FSH.
Letrozole can be used in patients working on getting pregnant with PCOS (or polycystic ovary syndrome) who have irregular or absent period.
Letrozole can be used as an alternative in women who develop a thin uterine lining on clomiphene citrate. Unlike clomiphene citrate, with letrozole there is no blockage of estrogen receptors and the uterine lining and cervical mucus are not affected.
Letrozole can be used in combination with fertility injections (gonadotropins) for IVF. This is especially so in patients with estrogen sensitive cancers where high E2 levels are to be avoided (see above).
Letrozole has also been used in poor responder patients along with high doses of gonadotropins.
The usual starting dose is 2.5 mg orally daily without regards to meals. It is usually given for 5 days starting on cycle days 2, 3, 4 or 5. The daily dose can be increased to 5 or 7.5 mg if needed.
Transvaginal follicle ultrasounds are the mainstay of cycle management. E2 levels remain low and are of very limited (if any) use.
Similar to those seen with clomiphene citrate (10 - 12% per cycle).
The most common side effects are sweating, hot flashes, joint pain and fatigue, nausea and diarrhea.
It is not approved for ovulation induction by the FDA. It is therefore used "off-label" in specific circumstances only.
Clomiphene citrate has now been used clinically for more than 40 years and remains the most common drug used for ovulation induction.
There is a controversy concerning birth defects with the use of Letrozole. The drug maker (Novartis) has sent a letter to physicians discouraging the use of Letrozole for ovulation induction. Recent data suggest that these fears are unfounded. This will be discussed in detail in my next blog.
To see a fertility specialist who is a board-certified physician with excellent success rates, make an appointment at one of InVia’s four Chicago area fertility clinics.

Entire Website © 2003 - 2020
Karande and Associates d/b/a InVia
Fertility Specialists
